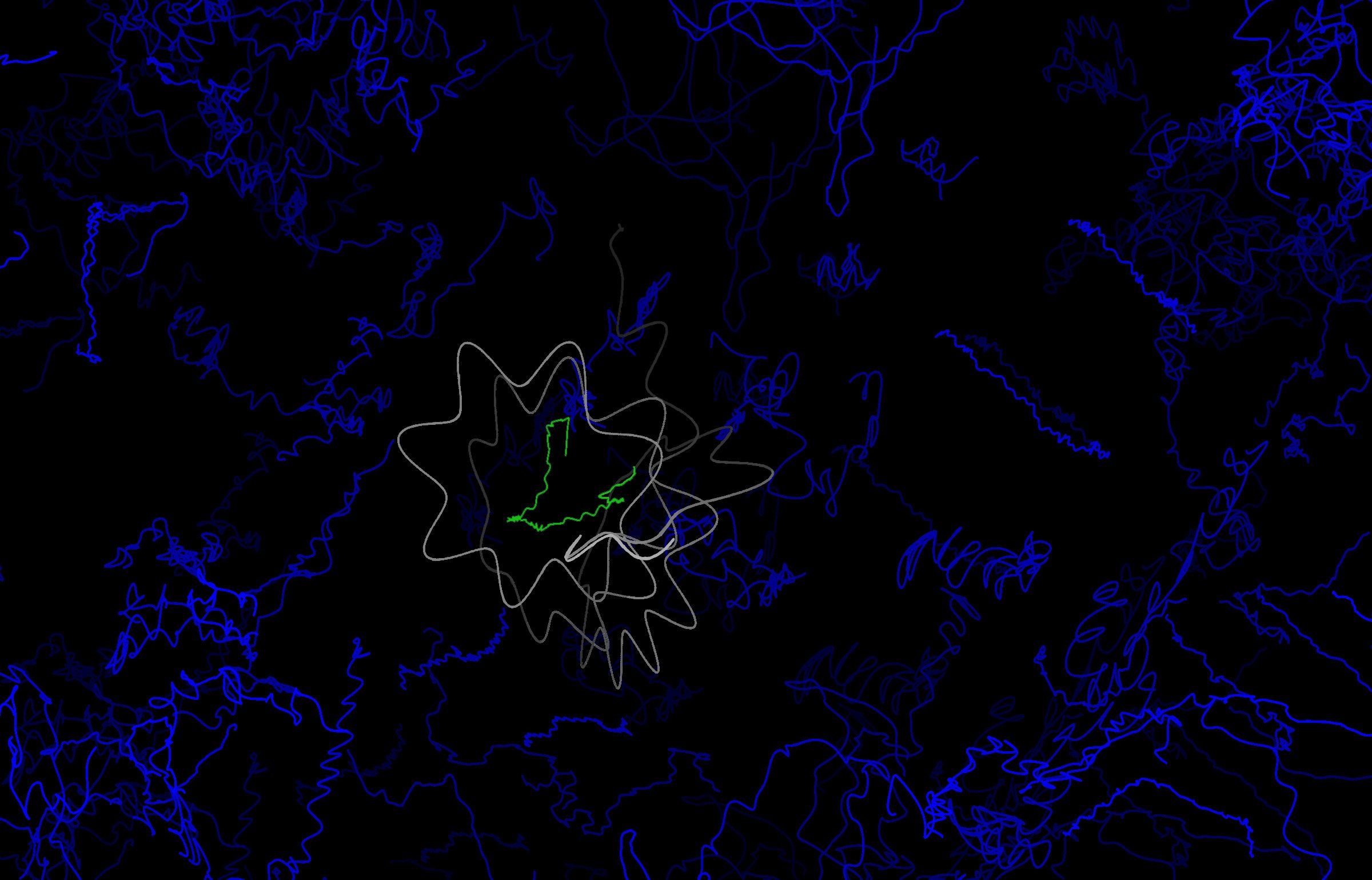
Results from a molecular dynamics simulations showing a 400-fs time trace of how the atomic nuclei of a DF molecules embedded in a liquid matrix move following the deposition of significant quantities of energy. Motion of the liquid is shown in blue, and motion of the DF in white. The interlaced starlike shapes arise from significant vibrational excitation in the newly formed DF
VISUALIZING Energy flow in liquids
The philosopher Thomas Berry famously wrote that “Awareness of an all-pervading mysterious energy articulated in the infinite variety of natural phenomena seems to be the primordial experience of human consciousness… an awesome universe filled with mysterious power.”
Our day-to-day experiences of energy are varied and beautiful: the clouds moving across the sky, the wind blowing through a tree, the unceasing waves crashing on the shore. The entire cosmos, at every level of organization, is characterized by energetic flux and perpetual motion. Richard Feynman famously said that ‘all of biology’ can be explained by the wigglings and jigglings of atoms.
At the tiniest level, string theorists postulate that different particles found in nature arise from the vibrational dynamics of tiny string-like objects. Moving up from strings to a slightly larger level, quantum mechanics tells us that matter actually exhibits wave-like behaviour with specific vibrations, energies, and frequencies. Microscopic living systems such as cells exhibit vibrations that last anywhere from milliseconds to seconds. And on the largest scale of all, cosmologists think that the entire universe is still vibrating at frequencies excited in the Big Bang. Microscopically, the movement of atoms depends on the energy fields that they experience from the atoms that surround them.
For some years now, the IRL has had an interest in making detailed ‘maps’ of how energy flows on the scale of atoms and molecules.
Two of these ‘energy mapping’ projects - which were specifically undertaken to understand how energy flows in liquids - have featured in the magazine “Science”. People have known for some time that if you deposit energy into a molecule using a laser light pulse, it can hold onto its energetic excitation for quite a while, despite the fact that it is embedded in a liquid. But most of the liquid chemistry occurring in nature happens under so-called “thermal” conditions, with no laser pulses involved. Under thermal conditions, the general assumption is that energy deposited in a molecule by way of a chemical reaction is dissipated so fast that it’s not really worth worrying about, and couldn’t possibly affect the reaction outcome.
Using a combination of ultrafast spectroscopy and state-of-the-art molecular dynamics simulations to examine simple chemical reactions in liquids, we have shown that – even under thermal conditions - the reaction products holds onto their energy for much longer than expected. One particularly surprising result is that this conclusion seems to hold even in so-called ‘strongly coupled’ systems, where we expected this NOT to be the case.
PUBLICATIONS
S.J. Greaves, R.A. Rose, T.A.A. Oliver, D.R. Glowacki, M.N.R. Ashfold, J.N. Harvey, I.P. Clark, G.M. Greetham, A.W. Parker, M. Towrie, A.J. Orr-Ewing “Vibrationally quantum-state-specific reaction dynamics of H atom abstraction by CN radical in solution,” Science, 2011: Vol. 331, no. 6023, p 1423-1426
D. R. Glowacki, R. A. Rose, S. J. Greaves, A. J. Orr-Ewing, J. N. Harvey, “Ultrafast energy flow in the wake of solution phase bimolecular reactions,” Nature Chemistry, 3, 850–855 (2011)
D. R. Glowacki, R. Lightfoot, J. N. Harvey, “Non-equilibrium phenomena and molecular reaction dynamics: mode space, energy space, and conformer space,” Molecular Physics, Vol 111(5), p 631, 2013
G.T. Dunning, D.R. Glowacki, T.J. Preston, S.J. Greaves, G.M. Greetham, I.P. Clark, M. Towrie, J.N. Harvey and A.J. Orr-Ewing, “Vibrational relaxation and micro-solvation of DF following F-atom reactions in polar solvents”, Science, Vol. 347 no. 6221 pp. 530-533 (2015)
B.K. Carpenter, J.N. Harvey, and D.R. Glowacki, “Prediction of Enhanced Solvent-Induced Enantioselectivity for a Ring Opening with a Bifurcating Reaction Path”, Physical Chemistry Chemical Physics, 2015, 17, 8372-8381